
In other words, the amount of force applied t.Īverage force can be explained as the amount of force exerted by the body moving at giv.Īngular displacement is the angle at which an object moves on a circular path. Torque is nothing but a rotational force. When an object falls into the ground due to planet's own gravitational force is known a. Here is a list of elements with their atomic mass which our molarity to parts per million converter uses while doing the calculation.Įnter the value of molarity in the below calculator and click calculate button to get the result in ppm.Īverage acceleration is the object's change in speed for a specific given time period. Our online molarity to PPM calculator will help you to convert more than 100 different types of elements from molarity to ppm considering the atomic mass of the typical element. Jan 2008 - 38 What is the concentration of O 2(g), in parts per million, in a solution that contains 0.008 gram of O 2(g) dissolved in 1000.Parts per million also known as PPM is a used for measuring the substance quantity per million parts of solution. grams of a solution having a concentration of 5 parts per million? The accuracy of our molar concentration depends on our choice of glassware, as well as the accuracy of the balance we use to measure out the solute. Step Stir until the is completely dissolved. Step Add water to the until the total volume of the solution is. (The molecular weight of TCE is 131.5 g.) Solution: In water, ppm is equivalent to, so we have 5.0 mg/l of TCE.

June 2009- 36 What is the total mass of solute in 1000. Step Transfer the sodium chloride to a clean, dry flask. Convert the concentration of TCE found in example 1.2 (5.0 ppm) to units of M (molarity). June 2008- 38 What is the concentration of O 2(g), in parts per million, in a solution that contains 0.008 gram of O 2(g) dissolved in 1000. Moles to Grams Calculator Common Compounds List Chemical Equation Balancer Complete List of Acids Complete List of Bases Molar to Mass Concentration Converter Molar Mass Calculator Cations, Anions List Dilution Calculator Molarity Calculator Compound Prefixes Water Insoluble Compounds Compound Quiz. June 2005 -42 What is the concentration of a solution, in parts per million, if 0.02 gram of Na 3PO 4 is dissolved in 1000 grams of water? grams of H 2O, what is the concentration of the resulting solution, in parts per million?
If 0.025 gram of Pb(NO 3) 2 is dissolved in 100. Here was another one, but it was a multiple choice.

It is technically wrong not to add the solute and solvents together to determine the mass of solution. Even though mathematically it didn't change a thing. Īnswer-The equation above reads "grams of solution", you had to add the solute to the solvent in the denominator. Your response must include both a correct numerical setup and the calculated result. Since the concentration of Compound A is 100 ppm, the total number of moles of Compound A in 1 liter is. In the video, the number of moles of barium present in a 1L sample of water with a barium concentration of 2ppm is calculated. In the space in your answer booklet, calculate the dissolved oxygen concentration of this solution in parts per million. One liter of gas will then contain (1/24.15 ) moles. An aqueous solution has 0.0070 gram of oxygen dissolved in 1000. Most students lost 1 of the 2 points on this one.Ħ6. Then June 2007, New York State threw this curveball. 100 ppm of dissolved O2 and you are asked to calculate the number of moles of O2. What is the concentration of a solution, in parts per million, if 0.02 gram of NaCl is dissolved in 1000. Moles C2H5OH 7.89 g × Molar Mass C2H5OH 7.89 g C2H5OH × 46.07 g/mol. This is really just a plug and chug problem. Think about this 1ppm = 1 inch in 16 miles This is used for very small concentrations of particles in solutions.
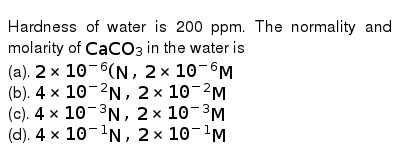
This is another way of determining concentration.


 0 kommentar(er)
0 kommentar(er)
